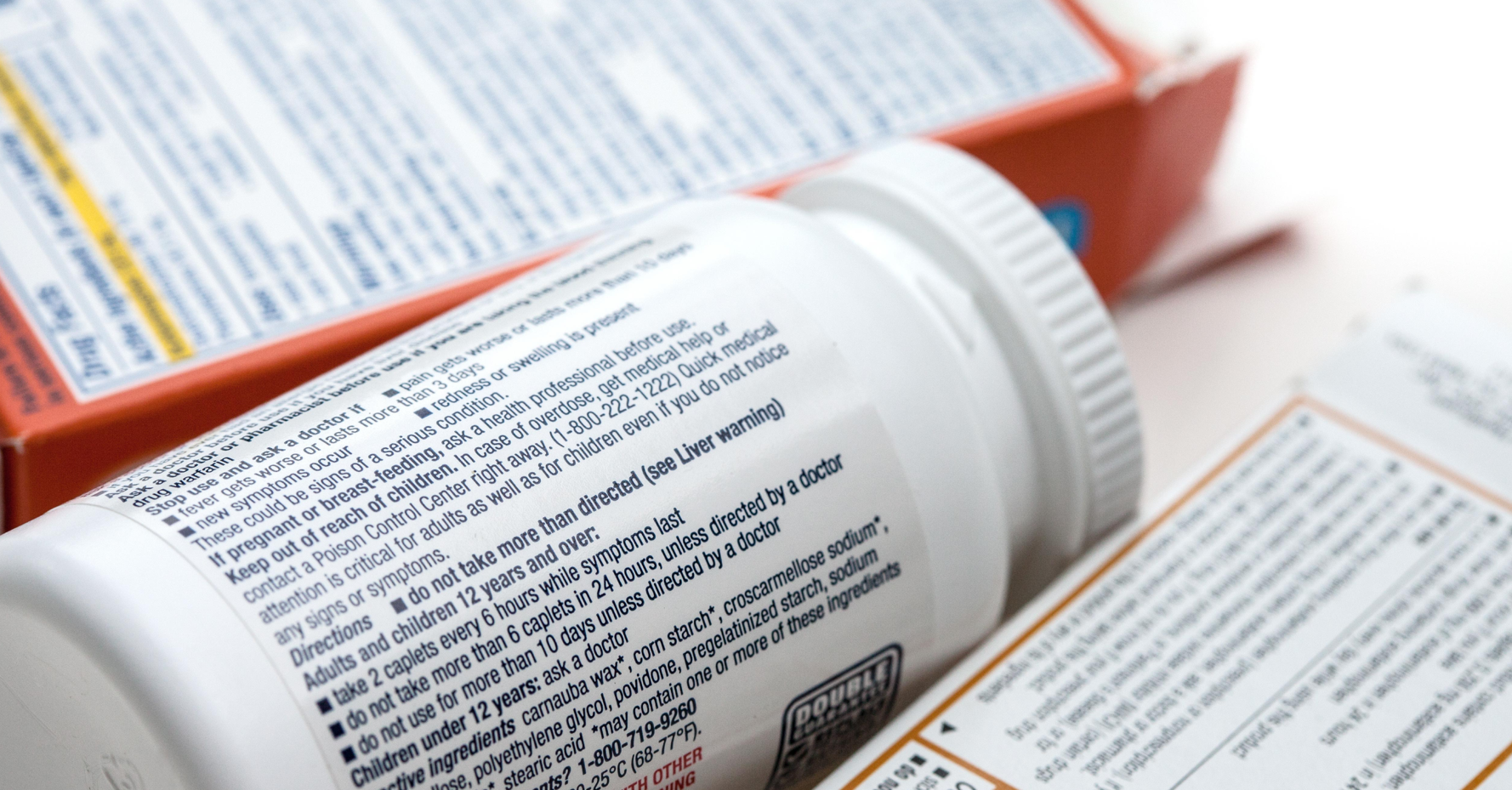
When it comes to labeling nutraceuticals and supplement products, the purpose extends far beyond eye-catching design—labels serve as critical tools for ensuring consumer safety, meeting stringent regulatory requirements, and maintaining traceability throughout the supply chain. They’re your frontline defense for patient safety, your ticket to regulatory approval, and your lifeline for tracking products through the supply chain.
For quality assurance managers and compliance officers, getting pharmaceutical labeling compliance right is a mission-critical part of the job. Whether you’re bringing a new product to market or updating an existing label, understanding the ins and outs of pharmaceutical labeling compliance will save you from miscommunications, delays, and expensive recalls, all of which can do long-term damage to your reputation and profits.
In short, what you don’t know can hurt you—so here’s what you should know.
Have questions? Our experts are here to help. Connect With a Consultant.
Pharmaceutical Labeling Leaves No Room for Error
Pharmaceutical labels carry life-critical information: drug names, dosage instructions, safety warnings, and batch numbers. Patients and healthcare providers rely on this information to make safe and informed decisions. A pharmaceutical labeling error can have serious (and possibly life-threatening) consequences for the consumer.
Add to that the complexity of global markets, where you might need to satisfy the FDA in the United States, the EMA in Europe, Health Canada, and other regulatory bodies, and you’ve got yourself quite a few hurdles to cross. Each has its own requirements, and navigating this regulatory maze requires precision and expertise.
That’s why accuracy, legibility, and tamper resistance are now legal requirements. GMP-certified label production, rigorous quality control, and a commitment to continuous improvement are all essential for compliance. For example, at Systems Graphics, we’ve built our processes around these pillars to ensure every label meets the highest standards for safety and reliability.
Understanding FDA 21 CFR Part 211 (and What It Means for Your Labels)
Let’s talk about the foundation of U.S. pharmaceutical labeling compliance: FDA 21 CFR Part 211. While the name itself is a bit of a mouthful, the concepts are not too hard to grasp once you know what they entail. This regulation defines current Good Manufacturing Practices (GMP) for finished drugs, and it has some very specific things to say about labeling:
The Must-Have Information
Every pharmaceutical container needs to clearly display several key pieces of information: the drug name, strength or potency, dosage form (think tablet, capsule, liquid), batch or lot number, and expiration date. Miss any of these, and you could be looking at a warning letter or product hold—neither of which you want on your record.
Ensuring Long-Term Legibility
Here’s a common sticking point: labels must be “legible and indelible.” That means your fonts need to meet specific size and contrast requirements, and the printed information has to survive whatever the product goes through during its shelf life. Any smudging, fading, or wear could make critical information unreadable.
Keeping Changes Under Control
Even small tweaks—adjusting a font size or updating a layout—require documented procedures for review, approval, and implementation. While this might sound like needless red tape, it’s actually your shield against avoidable confusion and mislabeling errors. This is as much to protect you as it is the consumer.
GMP Compliance in Action
Good Manufacturing Practice guidelines are all about three things: consistency, cleanliness, and documentation. For labeling, this translates into controlled artwork approval processes, documented material handling procedures, and careful label reconciliation to track usage and prevent problems like overprinting or unauthorized changes.
Facilities operating under strict GMP-certified protocols—with quality checkpoints, version control, and batch tracking—can significantly reduce risk and improve traceability during audits. Systems Graphics, for example, integrates these controls into every stage of label production.
Serialization and Traceability
As pharmaceutical supply chains have become increasingly complex, serialization requirements have evolved to match. In the United States, the Drug Supply Chain Security Act (DSCSA) now requires prescription drug packages to carry unique product identifiers.
Why does this matter? Serialization gives you several powerful capabilities:
- Complete track-and-trace visibility across your supply chain
- Robust counterfeit prevention through product authentication
- Rapid recall capabilities, since you can trace products by batch or individual unit
Variable data printing (VDP) enables the production of high-resolution, scannable barcodes and 2D codes at scale—an essential part of serialization. At Systems Graphics, our VDP systems are designed to handle large volumes of serialized SKUs efficiently, supporting compliance and supply chain agility.
Getting the Details Right: Language, Format, and Layout
Clear, consistent information presentation is non-negotiable in pharmaceutical labeling, especially when you’re serving multilingual markets. This means standardized formatting for drug facts and usage instructions, using font sizes and contrast levels that actually support readability and multi-language support for international sales.
Sometimes you need to include a lot of information without making your main label panel look cluttered. That’s where extended content labels, peel-and-reveal designs, and booklet formats really shine. Solutions like extended content labels, peel-and-reveal designs, and booklet formats—supported by precision die-cutting and digital proofing—help ensure even the most complex layouts remain compliant and readable. Systems Graphics provides these options to meet diverse client needs.
Handling Updates and Recalls
Product changes, regulatory updates, and manufacturing shifts can all trigger the need for urgent label revisions. Handle this process poorly, and you could end up with outdated or inaccurate labels in the field—a scenario that takes even more work to rectify.
Digital version control systems—such as Label Traxx—track artwork iterations, manage electronic approvals, and support batch-level identification for targeted recalls. Systems Graphics leverages these tools to help clients manage updates and maintain compliance.
If you do face a product recall, the traceability features built into our label systems allow you to quickly identify, locate, and recover affected units. This minimizes both risk and regulatory exposure when time is of the essence.
Finding the Right Partner Makes All the Difference
Pharmaceutical labeling compliance is undeniably complex, but it doesn’t have to become a constant source of stress and friction in your operations. When you work with Systems Graphics, you’re partnering with a team that truly understands the regulatory landscape, consistently delivers GMP-certified quality, and offers flexible solutions for everything from serialization to multilingual formats and complete label lifecycle management.
Whether you need help with a single SKU or you’re looking to convert an entire product line, we’ll make sure your labels support compliance from the moment they’re printed until they reach the shelf.
Ready to see how our pharmaceutical labeling solutions can meet your compliance needs with confidence? Connect with a consultant today.